What’s the best food for newly hatched fry? Finding a suitable food was one of my biggest difficulties when getting started with our club’s Breeders Award Program (BAP). Especially, since I was interested in spawning some of the smaller egglayers. Many BAP reports describe the use of live baby brine shrimp for rearing larger fry, but few mention how to rear smaller species to a sufficient size to accept this food. Smaller organisms, such as infusoria and “green-water” are occasionally described in aquarium literature, but not always in much detail. Since my initial attempt at culturing live microscopic food (by letting lettuce leaves decompose) wasn’t entirely successful as the process was pretty stinky and didn’t yield much food, I decided to look into this subject in more detail. Two excellent sources of information were found in an old T.F.H. book by Masters (i), which is available from our club library, and on the Internet (ii). The following article combines information from these sources with my own experience raising Paramecium cultures and I hope it will help others get started raising their own small fry.
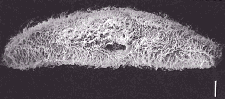
Figure 1: Paramecium multimicronucleatum photographed under a scanning electron microscope to show the many fine hairs (cilia) which cover its surface. The size bar is 10 mm. Photograph from M. J. Pelczar Jr. and R. D. Reid, Microbiology, 1972, McGraw-Hill Book Co.
The term “infusoria” is often used in aquarium literature to include all forms of microscopic life (animal and vegetable). Technically, however, infusoria are single-celled animals (protozoans) belonging to the family Ciliata and are quite different than the organisms raised in either “green-water” (single-celled algae and Euglena) or rotifer culture (multicellular invertebrates of the Rotifera). Infusoria were first observed in 1763 by microscopic examination of water, in which hay had been previously soaked. Masters estimates that there are 2,000 to 3,000 different species of infusorian protozoa and describes the genera Paramecium, Bursaria, Blepharisma, Stylonychia, Spirostomum, Volvox, Stentor, Vorticella, and Epistylis in his book (i). However, I doubt that many aquarists will ever find a need to individually identify these microorganisms (unless we can persuade our BAP coordinator to give us points for raising microbes).
The importance of infusoria to the aquarium hobby is with their culture for use as fry food. Their small size, ranging from 25 mm to 300 mm (a mm = 1/1000 of a mm) makes them an ideal live food for young fry which have just consumed their yolk sac. For comparison, brine shrimp nauplii are around 400 mm to 500 mm in size and are so big that many fry can’t consume them until at least a couple of weeks after hatching. Infusoria can be raised on quite a variety of foods. Originally hay was used, but almost any other source of vegetable matter, or even pablum, can be used. Basically, the food is briefly boiled (to help break down the tissue), cooled, placed in a large open-mouthed jar with water, and seeded with a starter culture. Masters provides half a dozen different recipes for culturing infusoria (i) in his book and similar, although less thorough, information can be found in other aquarium books, such as Andrews (iii), Scheurmann (iv), or Ramshorst (v).
Although infusoria are naturally abundant, collecting a suitable starter culture may require some work. When I first attempted my own culture, by letting a couple of lettuce leaves rot in a liter of water (iii), I believed that the culture would begin from microorganisms on either the leaves or introduced from the air. Although, there were certainly enough bacteria present to decompose the leaves, microscopic examination revealed very few protozoans and what I ended up feeding the fry was mostly water. This type of culture was very inefficient and the large amount of decaying vegetable matter was also unpleasant, and potentially harmful to the fry. It is probably much more efficient to begin an infusoria culture with a known source of material instead of relying on the chance introduction of some protozoans. A high population density of infusorians is required, especially when feeding large numbers of fry.

Figure 2: Paramecium general structure. From W. H. Adey and K. Loveland, 1991, Dynamic Aquaria, Academic Press, Inc.
Masters (i) suggests that infusoria for starter cultures can be collected from any stagnant pond where decaying vegetable matter is present, from the debris on established aquarium filters, from water in which cut flowers have been standing, or from the partly digested plant tissue in the droppings of infusorial snails (Ampullaria). However, with the exception of aquarium filter debris, most of these sources were unavailable to me during the winter. In addition, these sources all contained a mixture of many different microorganisms, some of which might not be desirable. A better way to culture live food is to begin with a pure strain, which can be purchased from commercial biological supply houses or obtained from local biology laboratories.
One particularly common type of infusoria, Paramecium, is frequently used in teaching and research laboratories. These unicellular organisms are among the most advanced protozoans and are characterized by the presence of many surface cilia (hairs) which are used for swimming and collecting food. These hairs are very fine and are only clearly seen under an electron microscope (Figure 1). Paramecia also have a very distinctive slipper-like appearance and range between 60 mm to 300 mm in length, with most around 150 mm. Under the microscope, these organisms have a semi-transparent appearance and the interior nuclei (there are two, a large macronucleus for ordinary cellular metabolism and a second micronucleus associated with reproduction) and food containing vacuoles are readily seen (Figure 2) as small globules. There are also distinct front (rounded) and rear (pointed) ends. Perhaps the most striking feature is the speed with which these tiny organisms can swim, especially when they don’t have any visible means of propulsion. It is a really amazing sight to see these little ovals zooming around in nonstop motion as they look for food.
From the Internet (ii), I learned that Paramecium cultures are in widespread use as fry food in the large scale laboratory rearing of zebra danios (Danio rerio) and so I decided to see if our local university could supply me with a starter culture. Fortunately, at that time the undergraduate biology labs were working with Paramecium caudatum and a friendly technician graciously took the time to provide me with a few mL’s of culture solution at no charge.
Culturing P. caudatum, like any other Paramecium, was quite straight forward. In accordance with Westerfield (ii), I decided to use wheat grains and yeast tablets as the source of food for these organisms (Figure 3). The wheat grains were purchased at a local bulk food store where an 800 g bag cost only $1.69. One hundred Brewer’s yeast tablets, fortified with vitamins (400 mg), were obtained from a drugstore for another $5. These tablets provided the essential vitamins while the wheat grains provided the main food source. The cost of purchasing these supplies was very minor since enough food for several years of culture was obtained. Many other food sources, including timothy hay, ground and dried lettuce powder, flour, or turnip (i, iv) can also be used but these are not as neat nor convenient.

Figure 3: Wheat grains and Brewer’s yeast tablets used to culture paramecia. Photo by R. T. Pon.
I used empty two liter plastic ice cream buckets as culture containers, although glass finger bowls and plastic mouse cages are used under actual laboratory conditions (ii). A culture was begun by placing about 20-30 wheat grains into a clean ice cream bucket and 1 cm to 2 cm of tap water was added. When my wife was out of the kitchen, the ice cream bucket was microwaved for about three minutes to bring the contents to a boil. This served to soften up the wheat grains and killed off any other microorganisms which might become contaminants. Cold tap water was then immediately added to fill the bucket about three quarters full. Approximately 1/4 to 1/3 of a yeast tablet was then snipped off with a pair of scissors and added to the bucket. A few mL’s of an existing culture (I started with about 10 mL) of Paramecium caudatum was added and the new culture was loosely covered with a piece of aluminum foil and left to sit. The ideal incubation temperature is 28.5C although room temperature also works. My fish room is kept around 25C and if I want to get a culture going a little faster, I place the pail on top of a wooden light hood where it warms up to about 28C. As the culture reproduces, the water will gradually become cloudy and a small amount of scum will form.
The biggest problem the home aquarist will have is determining how well the culture is doing. If a low power microscope is available, then the culture’s progress can be easily monitored by examining a drop of liquid under 50X magnification (Figure 4). A culture should have several fully grown paramecia swimming around in the field of view and a good culture will be swarming with paramecia. There are many more organisms in a successful culture than I have ever observed with unseeded infusoria attempts, and this makes the trouble obtaining a seed culture well worth it. Population growth can take between four to fourteen days to occur, depending upon the temperature.
If a microscope is not available, then a hand held magnifying glass can also be used, but then the full-grown paramecia will be a lot harder to see and smaller ones will be undetectable. Remove only a drop or two of liquid from the culture and place it on a piece of glass. The paramecia will be visible as tiny slivers of movement around the edges. Remember that you’re looking for something only about one-tenth of a mm long. After about three to four weeks, the cloudy culture will become clear and this indicates that it’s time to start a new batch. Use a few mL’s of the bottom muck as starter culture, and be sure to include a couple of old wheat grains to ensure the introduction of enough paramecia.
Another problem is an unpleasant odor that may sometimes develop. I’m not sure what causes this odor, since it doesn’t always occur, but it may be related to having too many wheat grains and yeast, or not enough aeration. Masters (i) warns that while “There is no single method to be used universally for successful culturing It is only important to remember that SMALL quantities of materials, usually organic, must be put into water…[as] the chief food supply of paramecia”. However, keeping a loose aluminum foil cover over the culture greatly helps to contain the smell and after a few days most of the smell goes away.

Figure 4: Live P. caudatum as seen under a microscope. 150X magnification. Photo by R. T. Pon.
It is advisable to keep at least a couple of different cultures operating at the same time (more if you have a lot of fish spawning or about to spawn) to ensure that there is always a strong culture available for feeding. Storage of a back-up culture in a second, cooler location also provides some insurance against failure of the primary cultures. I have been keeping Paramecium cultures for about six months now and still find it difficult to predict exactly when and for how long a culture will remain good. To the naked eye, the solutions all look the same (just cloudy, slightly brown water) and the only way to know how well they’re doing is to examine them with a microscope or magnifying glass. This only takes a few moments and it guarantees that you’re actually giving the fry liquid with something in it.
Feeding paramecia is a very simple operation. Once the fry have absorbed their yolk sacs and become active swimmers (about two to four days after hatching, depending on the species), samples of the culture liquid can be added directly to the fry tank. I use a glass turkey baster (30 mL capacity) to remove liquid from just under the surface scum of the culture. I try to avoid the sticky slime which forms on the bottom of the bucket. Somehow, by sight or smell, the small fry are able to detect the presence of the microscopic food. One can soon see the fry making quick jerking movements as they capture the protozoans. It’s difficult to know exactly how much to feed since the food can’t be seen in the tank. Masters (i) suggests that 30 mL is sufficient for fifty fry in a 20-liter tank while others (ii) simply say to feed large quantities. I prefer to err on the surplus side and I usually add a squirt or two (30mL to 60 mL) to the tank, up to four times per day. Since this is a live food, normally found in fresh water, excess paramecia won’t die and foul the water. This fact, along with the essential vitamins and nutrients contained in live food, are major benefits to using these cultures.
Some references will recommend separating the organisms from the excess food by either filtering through filter paper (ii) or silk gauze (iv) to prevent the culture’s food from going into the aquarium. However, this is probably only necessary when raising fry in very small, crowded, and unfiltered containers, such as 250 mL beakers. I have never had any problems using a 20-liter spawning tank equipped with a sponge filter. When the volume of my culture gets low, I even suck up a few of the decomposing wheat grains and other scum and add them to the fry tank. It doesn’t look pretty but it’s never done any harm, and the fry soon learn to feed off the surface of these wheat grains.
Using this procedure, I have been able to raise leopard and zebra danio fry as well as cherry, gold, and Odessa barbs. These fry, especially the cherry barbs, are all very small and it is vital that they be given a suitable food as soon as possible. My hatch rates have all been pretty good and with the gold and Odessa Barbs: 300 to 400 fry have been raised at a time. In each case, Paramecium culture was added directly to the fry tank as the sole food for the first couple of weeks and in conjunction with live brine shrimp nauplii for another week or so, until all the fry were easily consuming the nauplii.
The use of Paramecium cultures to raise fry has become widespread throughout the scientific community because of the importance of zebra danios as laboratory animals, and this culture has also proven itself to be very useful in my own fishroom. Unfortunately, like many other aspects of the aquarium hobby, accurate information about this subject is not always easy to find and I would like to point out a couple of instances which may cause unnecessary confusion.
First, in Aquarium Fish Breeding, Scheurmann gives the impression that paramecia can only be raised on a particular species of turnip (Brassica napus) and that “Delicate fry … cannot tolerate the bacteria in the water of the [Paramecium] breeding jar” (iv). This reference then goes on to describe a complicated method for separating the paramecia from the bacteria using a long (50 cm) tube. Certainly Paramecium can be cultured on a much wider range of materials than the turnip mentioned and I feel that the purification procedure is cumbersome, inefficient, and unnecessary. Good fish keeping practice (clean water and clean tanks, etc.) are probably much more important. Secondly, in the otherwise excellent Aquarium Atlas by Riehl and Baensch (vi), there is a glaring mistake in their discussion of microscopic food. Paramecium is not only described as a form of plankton, which it is not, but further described as “these sting and are scorned by fry”. There is absolutely no stinging mechanism in these organisms and one must wonder if the authors were confusing Paramecium with Hydra or some other nasty organism.
I hope that this article will help clarify the culturing of live microscopic fry food. The set-up and maintenance of these cultures is really quite easy and I’ve had excellent results rearing fry. Someday, I might to try use infusoria from one of my filter pads as a seed culture, but for now my P. caudatum culture seems to be continuing quite well. Anyone interested in more information about live foods, both micro and macroscopic, is strongly encouraged to check The Encyclopedia of Live Foods (i) which our club’s library has available. I am also willing to supply starter cultures of my paramecia to any CAS member who wants some.
REFERENCES
i) Charles O. Masters, 1975, Encyclopedia of Live Foods, T.F.H. Publications Inc.
ii) Monte Westerfield, 1995, The Zebrafish Book. A guide for the laboratory use of zebrafish , Third ed., University of Oregon. Available at no charge off the Internet from http://zfish.uoregon.edu/zf_info/zfbook/zfbk.html.
iii) Chris Andrews, 1986, A Fishkeeper’s Guide to Fish Breeding, Tetra Press.
iv) Ines Scheurmann, 1990, Aquarium Fish Breeding, Barron’s Educational Series Inc.
v) J. D. van Ramshorst ed., 1991, The Complete Aquarium Encyclopedia of Tropical Freshwater Fish, Bookmart Ltd.
vi) Rudiger Riehl and Hans A. Baensch, 1994, Aquarium Atlas, 4th ed. Tetra Sales. ?
